 * The 2021 Version of an RSR Classic: Carbon-14 doesn't lie. It has a relatively brief life and yet is plentiful in fossils and diamonds claimed to be millions and billions of years old. Carbon-14 can't be an anomaly, because it's not here and there but it's everywhere it shouldn't be. This radiocarbon is so unstable that a solid ball of 14c the size of the Earth would all decay into nitrogen in less than a million years. Real Science Radio hosts Bob Enyart and Fred Williams begin today's Carbon-14 program by airing Bob's phone conservation from 15 years ago with Jurassic Park film advisor Jack Horner. Bob offered Jack $23,000 to carbon date the soft tissue T. rex that his team had recently excavated. (The 14c argument for a young-earth parallels the argument made from the 50 peer-reviewed scientific papers, listed at bflist.rsr.org, documenting the existence of still-soft dinosaur blood and many other examples of dinosaur biomaterial "fossils".)
* The 2021 Version of an RSR Classic: Carbon-14 doesn't lie. It has a relatively brief life and yet is plentiful in fossils and diamonds claimed to be millions and billions of years old. Carbon-14 can't be an anomaly, because it's not here and there but it's everywhere it shouldn't be. This radiocarbon is so unstable that a solid ball of 14c the size of the Earth would all decay into nitrogen in less than a million years. Real Science Radio hosts Bob Enyart and Fred Williams begin today's Carbon-14 program by airing Bob's phone conservation from 15 years ago with Jurassic Park film advisor Jack Horner. Bob offered Jack $23,000 to carbon date the soft tissue T. rex that his team had recently excavated. (The 14c argument for a young-earth parallels the argument made from the 50 peer-reviewed scientific papers, listed at bflist.rsr.org, documenting the existence of still-soft dinosaur blood and many other examples of dinosaur biomaterial "fossils".)
* RSR's 2021 Carbon 14 Series: Pt. 1 (today's show), Pt. 2, Pt. 3, Pt. 4, and Pt. 5.
 * Video Subscription: To order a monthly video subscription, just call 800-8Enyart or click here to receive our monthly:
* Video Subscription: To order a monthly video subscription, just call 800-8Enyart or click here to receive our monthly:
- Topical Videos
- Sermon Videos
- Bible Study Videos
- BEL Televised Classics
* 14c Factoids: For a better "gut feel" for the topic...
- Our atmosphere has one carbon-14 atom for every one trillion carbon atoms.
- 21 pounds of 14c are produced in the atmosphere every year!
- Unstable radiocarbon decays with a half-life of 5,730 years. (half-life symbol = t1/2)
- A solid ball of 14c the size of the Earth would all decay into nitrogen in less than a million years.
- A gram of carbon has about 50 sextillion carbon atoms. (50,000,000,000,000,000,000,000)
- RSR co-host Fred has about 10 octillion total atoms in his body. (one octillion of carbon)
- Fred, a big guy, carries around about 50 billion radiocarbon atoms! (50,000,000,000)
- Fred's body experiences about 2,500 decays of 14c to nitrogen every second.
- One 14c atom in a trillion carbon atoms is referred to as 100 percent modern carbon. (100 pMC)
- Likewise, 100 14c atoms in a 100 trillion is referred to as 100 percent modern carbon.
- So 50 14c atoms in a 100 trillion carbon atoms is referred to as 50 percent modern carbon. (50 pMC)
- Fifty percent modern carbon is interpreted as 5,730 years before present. (about 6,000 years BP)
- The best AMS labs accurately count 14c atoms to even 0.001 of a percent modern carbon. (100th to a 1000th pMC)
- Published lab measurements show processing introduces 1/10th to 3/10ths of a percent contamination. (~0.2 pMC)
- So lab contamination adds one 14c atom to 250 in 500 trillion carbon atoms. (Yielding 251 of 500 trillion or 50.2 pMC.)
- 14c "ages" get progressively significantly inflated as with bones from 873 AD dated to 200 years earlier.
- Pompeii, destroyed in 79 AD, was expected to give appropriate ages but gave 2400 to 5800 years BP.
- Old 14c "dates" are progressively too old and there's far too much C-14 in diamonds, etc., to be contamination!
* Telethon: The guys ask for help with their telethon goal of $50,000. If you can, please check out kgov.com/store or call us at 1-800-8Enyart (836-9278) to purchase our science resources, sign up for a subscription, or to make a one-time or monthly donation. Thanks so much!
* Correction: As reported already back in 1962 and 1984, plants do absorb carbon dioxide from their roots, but at 27:20 into today's program Bob unintentionally implied that was the only way. Of course most CO2 is abosbed through leaf stomata, pores that form typically on the underside of leaves. Also, at 25:45 the guys are talking about Prof. Steve Taylor of the University of Liverpool.
* Time-Saving Google Creation Tool: Multiple Creation Site Search!
* Full Report: Click to read our full Carbon-14 page.
* Carbon-14 is Everywhere It Shouldn't Be: Because of its short half-life, if plenty of 14c in quantities far above a modern lab's margin of processing and measurement errors, is found everywhere and in specimens allegedly millions or billions of years old, that is powerful hard scientific evidence that these are not millions but only thousands of years old. For a typical sample, all of its original 14c atoms would radioactively decay in far less than 100,000 years. Theoretically, contamination, measurement error, or neutron capture could explain apparent radiocarbon in allegedly older samples. On these, see below, but briefly for example, while modern bacteria can infest and bore its way through a dinosaur bone, in 2017 Nature reported an isotope study that found that such bacteria does not affect the bone's radiocarbon content (and date) because the carbon the bacteria feeds on does not come from the atmosphere but comes exclusively from the bone itself! Thus radiocarbon dating gives a relatively young upper age for specimens regardless of the primary contamination concern, namely, bacteria.
It has a relatively brief life and yet is plentiful in fossils and diamonds claimed to be millions and billions of years old. (See bflist.rsr.org for a list of dinosaur biomaterial fossil papers.)
Anything even just one million years old should have no "modern" carbon (once the well studied background contamination is subtracted). However, scientists are consistently finding significant amounts of 14c everywhere it shouldn't be including the 2019 report in eLife on a Centrosaurus. In 2011 the journal PLoS One reported plenty of modern carbon in an allegedly 80-million year old mosasaur bone. The journal Radiocarbon has reported 14c in natural gas, coal, oil and other petroleum products. Other careful studies report radiocarbon in limestone (from the Mesozoic layer), fossilized wood, coal, marble, deep groundwater, geological graphite, and in many dinosaur bones including the ten described below and the seven described in 2015 in the Radiocarbon in Dinosaur and Other Fossils paper by paleobiochemist Dr. Brian Thomas and his co-author Vance Nelson. And as reported at ScienceDirect, short-lived 14c is regularly found even in supposedly billion-year-old diamonds! The assumption by evolutionary geophysicists proposes that the 14c in diamonds, coal, etc., must have come from neutron capture by carbon-13 or nitrogen-14. Theoretical physicist Lawrence Krauss told RSR's Bob Enyart (rsr.org/krauss) that 14c in allegedly million-year-old specimens is an "anomaly." However, an anomaly is something that deviates from what is standard, normal, or expected. Because radiocarbon exists in significant quantities, far above the least count (margin of error) with our state-of-the-art AMS labs doing the tests, these results can no longer be called anomalies! The Radiocarbon field itself now widely acknowledges, and tries almost desperately to discount, that specimens supposedly millions and billions of years old will yield maximum carbon-14 ages of only thousands of years!
* Carbon 14 in Dinosaurs at Singapore's American Geophysical Conference: On how to date a dinosaur, Real Science Radio's Bob Enyart interviews Hugh Miller, a member of the international scientific team that presented at the 2012 AGU geophysical conference in Singapore, the carbon dating results from five respected laboratories around the world of bones from ten dinosaurs (from the Gobi Desert in China, from Europe, Alaska, Texas, and Montana). 14c lasts only thousands of years, not millions. Yet each of these dinosaurs had plenty of radiocarbon (as expected in that virtually every relevant peer-reviewed paper on the topic confirms the presence of endogenous soft tissue in fossils; see DinosaurSoftTissue.com). With the scientific breakthroughs and discoveries coming in daily, this is a great time to be alive!
* Update: At creation.com/c14-dinos, see the great summary of this presentation from Carl Wieland, president of Creation Ministries International. And consider this from a peer-reviewed paper in a respected scientific journal, "at a constant 10°C (the approximate mean annual air temperature in Britain today) it will take between 0.2 and 0.7 Ma for levels of collagen to fall to 1% of their original concentration in an optimal burial environment."
* Getting Graded: An expert on radiocarbon dating, long-time assistant professor at Loma Linda University, Dr. Paul Giem himself, graded the information presented below. (You can hear that interview with Dr. Giem here). The teacher corrected a couple points and clarified a few others. He gave the original text (available here) a grade of A minus. Of course we're hoping that now that we've corrected the material below, that this is solid A+ work!
 * Six Problems with the Contamination Explanation: (See the 6th point below for why collagen itself cannot be contaminated with modern carbon and for the Nature 2017 paper explaining that microbes like cyanobacteria in fossils get their carbon not from the atmosphere but from their bone substrate, meaning that they cannot supply a younger date than a dinosaur fossil, because they date the same as that fossil!) Both mathematical analysis of the data, and the nature of some of the specimens, indicate that contamination does not solve the radiocarbon problem for old-earth geologists.
* Six Problems with the Contamination Explanation: (See the 6th point below for why collagen itself cannot be contaminated with modern carbon and for the Nature 2017 paper explaining that microbes like cyanobacteria in fossils get their carbon not from the atmosphere but from their bone substrate, meaning that they cannot supply a younger date than a dinosaur fossil, because they date the same as that fossil!) Both mathematical analysis of the data, and the nature of some of the specimens, indicate that contamination does not solve the radiocarbon problem for old-earth geologists.
First: While dinosaur bones, coal, and other specimens could easily be contaminated, diamonds, the hardest naturally occurring substance in the world are naturally resistant to contamination. Thus, when significant quantities of 14c are found, for example, in coal and dinosaur bones, as well as in diamonds, the least contamination-resistant 14c-rich specimens provide a constraint on the likelihood of contamination as a primary source for the modern carbon in other similarly-dated specimens. Thus the radiocarbon content of diamonds is all the more compelling and important, and especially when the specimens are mined from a quarter-of-a-mile below the surface, insulated from our 14c-bearing atmosphere. Contamination is not only far more unlikely within deep-mined and unbroken diamonds, but because of the unique physical composition of diamonds, various kinds of contamination could be more readily detectable.
Second: Just as forensic accountants can often determine when a criminal business has cooked its books merely by doing a Benford statistical analysis of the numbers, so too mathematicians have demonstrated that statistical analysis can indicate whether scientific data is likely a result of measurement errors. So evolutionists typically claim that all this 14c results from contamination, but statistical analysis indicates that when plotting erroneous dates (as from contamination), the data should fit a normal curve. However, it does not. As documented by Rick Sanders in A Case of "Missing" Decay in CRSQ, the distribution provides significant evidence that the radiometric ages do not result from contamination errors. Regarding the results from the ten dinosaurs dated (as above), of course, bacteria do not make collagen. And if the 14c came from contamination, for example, one would not expect the contamination to so equally affect the bioapatite and the collagen.
Third: The above paper indicates that where sampled, the ground area has decreasing 14c with distance from the dinosaur bone, indicating that modern carbon is leaching out of the bone (which is not problematic), but, most significantly, not seeping into the bone.
Fourth: Dinosaur bone showing 5pmc means that five percent of the carbon in the bone needs to be replaced with modern carbon, which high level of contamination would very possibly be detectable.
Fifth: Dinosaur bone showing 5pmc means that, because the 14c half-life is so brief, 5,730 years, if the radiocarbon is from contamination that occurred 6,000 years ago, a full five percent, i.e., 1/20th of the bone must have been replaced. (See below, 42 minutes into Paul Giem's 2013 presentation.) If the contamination occurred 12,000 years ago, 10% of the bone would have to be replaced; 18,000 years ago, 20%; if it occurred 24,000 years ago, 40% of the bone would have had to be replaced, and if (in the evolutionary perspective) a mere 30,000 years ago, 80% of the bone would have to be replace by a contamination process. Thus, where researchers find both soft tissue and 14c, especially in small bones as with the mosasaur, the claim that the biological tissue is dinosaurian and is not contamination works exactly against the claim that the 14c is from contamination.
Sixth: The inventor of the radiocarbon dating method, Dr. Walter Libby, stated in the journal Science, "There is no known natural mechanism by which collagen may be altered to yield a false age." To clarify (and still, as of 2021) there is still no known mechanism to contaminate collagen with modern carbon formed in the atmosphere. Further, a 2017 paper in Nature, Carbon fixation from mineral carbonates, confirms that cynobacteria in fossils get "virtually all" of their carbon from the bone substrate they are feeding on! Therefore they cannot "contaminate" the 14c results because they will carbon "date" the same as the bone itself, for their percentage of modern carbon is identical to that of the bone. (Further, there is a pretreatment process of repeated washes of acid/alkali/acid to remove any outer humic acid and debris.) Therefore creationists have been correct to dispute Mary Schweitzer, Lindgren, et al., as they've tried to explain away as microbial contamination the "modern" carbon in an endogenous biomaterial Mosasaur bone. Etc. (See also this post from a committed evolutionist in a battle royale with our old friend rsr.org/david-willis at the Evolution Fairytale forum run by RSR host Fred Williams.) Regarding Libby's "no known natural mechanism" way of contaminating collagen, here's our RSR explanation of why this is. If a specimen is purified to 95% collagen, or 98%, or 99%, etc., then approximately the same percent of the carbon in the fossil sample will be endogenous (i.e., original to the living animal). Why? Because in collagen, new carbon atoms cannot replace original carbon atoms in the tightly-woven scaffolding molecule. As a result of decomposition, to the extent that original carbon atoms were decaying into a gas (nitrogen) and thereby falling out of the scaffolding, then to that extent you would no longer have collagen; rather, to that extent you would have humic acid. Decomposing collagen cannot be "repaired" by free carbon atoms happening upon the decomposition. Rather, the collagen must be manufactured within a living animal (with its constituent carbon atoms) into a "super-super-coil... interdigitated with its neighboring microfibrils... so well ordered as to be crystalline." Further, bacteria do not make collagen, which eliminates another possible source of contamination. So if a researcher can verify that he has a sample that has been purified to 99% collagen, for example,  then he can be sure that all the carbon in that 99% of the sample is original.
then he can be sure that all the carbon in that 99% of the sample is original.
2021 Update - Technology vs Old-Earthers: A form of electron microscopy called ptychography captures high resolution images of individual atoms (see right) at 100 million times magnification! If one day scientists using advanced technology can confirm a 100% pure collagen sample, that will end the claim that its short-lived carbon-14 might be contamination!
* Six Problems with the Neutron Capture Explanation
First: Unexpected C14 is found in specimens worldwide, yet 14c production (in the ground as compared to in the atmosphere) requires a lot of nearby radioactivity to produce appreciable amounts of 14c by neutron capture. However, terrestrial radioactivity is concentrated, with the vast majority of it occurring in the continental crust. (On RSR Lawrence Krauss confirmed this well-documented observation.) Ninety percent of Earth's radioactivity is in 1/3rd of 1% of it's mass.
Second: Radioactivity is relatively scarce even in the continental crust, at least as documented by this U.S.G.S. report for enormous swaths of land.
Third: Presented at the 2012 AGU Singapore conference, there was less than 20 parts per million of uranium and thorium in the dinosaur bones that contained large quantities of modern carbon, so much that it registered mid-range in the AMS (accelerator mass spectrometry) capabilities. Also, Uranium mines where the uranium content is 18% yield carbon specimens which have 1% 14c.
Fourth: In a meeting with RSR, a geologist with a degree from Colorado's School of Mines who has a background in nuclear physics (who also spent years bombarding various elements with neutrons to make isotopes for industry) told RSR that Carbon does not easily absorb neutrons because it is the heavier elements beginning with Sodium that readily capture neutrons. Further, while it is relatively unlikely that a Carbon atom will capture a free neutron, industrial processes use Carbon to slow down neutrons, whereas they use heavier elements, typically starting with Silicon, which is almost double the atomic weight of Carbon, for neutron capture. Creating 14c from Nitrogen, then, has essentially the same problem, because Carbon and Nitrogen are neighbors on the periodic table.
Fifth: Dr. Paul Giem writes that, "since nitrogen creates carbon-14 from neutrons 110,000 times more easily than does carbon," samples with even tiny amounts of nitrogen would dramatically increase carbon dates, such that, "If neutron capture is a significant source of carbon-14 in a given sample, radiocarbon dates should vary wildly with the nitrogen content of the sample." Giem adds, "I know of no such data."
Sixth: Recognizing that crustal radioactivity is generally relatively scare (as documented in this U.S.G.S report for coal, basalt, shales, granite, fly ash, etc.), Dr. Jonathan Sarfati builds upon Dr. Giem's research arguing that neutron capture could account for less than one 10,000th of the C-14 in diamonds (see these peer-reviewed calculations). Therefore, there would have to be thousands of times more uranium, thorium, etc. throughout the earth's crust everywhere that these globally dispersed materials are found.
* RSR Proposed Contamination Falsification Experiments: In addition to work already done documenting appreciable 14c levels even in contamination-resistant specimens, we recommend a few experiments including a couple proposed by RSR friend David Willis:
1) Obtain newly recovered and carefully protected dinosaur bones excavated from deeply-buried strata.
2) Confirm that the bones do not have appreciable quantities of radioactive material.
3) Then radiocarbon date specimens extracted from twenty locations within a single bone, including from the surfaces, near the surfaces, and midway toward the center, and from the center of the bone. The young earth model predicts the finding of significant quantities of carbon 14 throughout the bone. The evolutionary model would predict no modern carbon in such a bone, but as a secondary assumption, if 14c is found, since any contaminating material would have to pass through the outer layers of the bone to get into the center, the contamination explanation would expect to measure generally decreasing percentages of 14c from the outside to the center of each individual bone.
4) Repeat.
A second experiment, beginning as above, would be to date a small diameter bone and a larger diameter bone from the same dinosaur. Getting the same dates would help rule out contamination because the smaller bone will have a larger surface to volume ratio which, if contamination were a significant factor, should result in higher percentages of modern carbon.
A third experiment that could falsify contamination as a possible source of 14c involves:
1) Obtain diamonds from the Popigai Astroblem mine. With the announcement that these reserves in Siberia contain diamonds that are "twice as hard as normal", these will be ideal for 14c dating because their natural hardness would further rule out contamination. Also, evolutionary geologists claim that these diamonds were already ancient when, allegedly 35 million years ago, a meteor impacted above them.
2) Confirm that the diamonds do not contain appreciable quantities of radioactive material.
3) Radiocarbon date the Popigai Astroblem diamonds (including specimens near the surface and at the center). The evolutionary model predicts no (carbon dead) 14c. The young earth model predicts significant quantities of 14c measurable throughout the diamond.
4) Repeat.
A fourth experiment that could falsify contamination as a possible source of 14c involves radiocarbon dating of allegedly 100 million-year-old amber, by selecting pristine specimens, the condition of which may also help to rule out contamination.
A fifth experiment that could falsify contamination as a possible source of 14c involves on-location dating using the portable 14c device being created by a team at Liverpool University including RSR friend Steve Taylor. (Hear Bob's interview with Steve. Also, at the 7th ICC Dr. Taylor played Bob Enyart's phone call to Jack Horner offering a grant of $23,000 to carbon date his soft-tissue T. rex.) Dating bones in situ, and dating their surrounding matrix, will elimate various sources of possible contamination and provide significant additional data.
A sixth experiment would use an a not-yet-invented technology or perhaps the electron ptychography microscopy described above to carbon date a 100% pure collagen sample!
* RSR Proposed Neutron Capture Falsification Study: Published by RSR on Aug. 25, 2012. Ninety percent of Earth's radioactivity is concentrated in 1/3rd of 1% of it's mass (within the continental crust). Consider then, the fossil remains of organisms that had lived near the surface but that have been long buried in ocean sediments. If neutron capture were responsible for much of the unexpected 14c, then collectively, such ocean specimens, collectively, should have far less 14c than specimens excavated on the continents. Further, more analysis should be done on relevant specimens excavated from uranium mines, comparing their radiocarbon percentages to similar (in type and estimated date) specimens gathered away from uranium mines. Thus, marine deposit specimens and uranium mine specimens can function as control groups.
* What About Carbon 14 Dates Older than Genesis?: Carbon 14 dating involves major unsubstantiated assumptions, including the stability of the 12c/14c ratio over millennia, which the public is almost universally unaware of. Regardless though, for an uncontaminated specimen (like diamonds, pure collagen, dinosaur soft tissue, etc.), the isotope's rapid decay rate can disprove any claimed age of over 100,000 years. Out of every trillion Carbon atoms in the atmosphere, only about one is 14c. Thus a small change in this ratio can generate invalid older dates. Further, significant unknowns, both in the rates of 14c production in the atmosphere, and in the Earth's enormous geologic upheaval in the past, could have altered the 1,000,000,000,000-to-1 ratio.  For example, as reported on RSR, University of Maryland geophysicist Daniel Lathrop stated that, "over the last 150 years or so, the Earth’s magnetic field has declined in strength about ten percent..." And a stronger magnetic field thousands of years ago would result in production of fewer 14c atoms, which would result in older-than-actual 14c dates, as explained on RSR by Dr. Henry Richter, the NASA scientist who launched our first satellite, oversaw development of the scientific equipment used on the first lunar missions, and who played an important role in the early discovery of the Van Allen radiation belt. By the way, Dr. Richter also wrote the NASA publication Space Measurements Survey: Instruments and Spacecraft covering the great period in the history of science from October 1957 to March 1965.
For example, as reported on RSR, University of Maryland geophysicist Daniel Lathrop stated that, "over the last 150 years or so, the Earth’s magnetic field has declined in strength about ten percent..." And a stronger magnetic field thousands of years ago would result in production of fewer 14c atoms, which would result in older-than-actual 14c dates, as explained on RSR by Dr. Henry Richter, the NASA scientist who launched our first satellite, oversaw development of the scientific equipment used on the first lunar missions, and who played an important role in the early discovery of the Van Allen radiation belt. By the way, Dr. Richter also wrote the NASA publication Space Measurements Survey: Instruments and Spacecraft covering the great period in the history of science from October 1957 to March 1965.
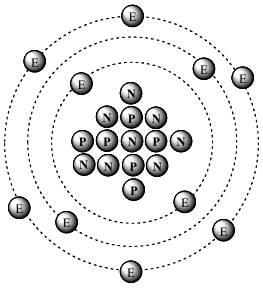
* Less Familiar Sources of 14C: Though it may be an extremely negligible source, of the various emissions from radioactive decay (alpha particles, beta particles, etc.), from a particular decay chain, it is possible that an entire 14c atom (6P, 8N, 6E) can be emitted as a unit in a single step. (If you know of a web page where this is described, please email that link to Bob@kgov.com. Thanks!) Also, as only widely recognized in 2017, lightning can produce 14c. See rsr.org/origin-of-earths-radioactivity.
* 14C in Other Dinosaur-layer Bones: RSR offered famed paleontologists Jack Horner and Dr. Mary Schweitzer a grant of $23,000 to carbon date their biological dinosaur tissue (YouTube video below), which money RSR saved thanks to Hugh Miller (above) and the rare studies that are just beginning to be reported finding significant quantities of modern carbon in allegedly ancient fossils that still contain original soft tissue! A Mosasaur shown by researchers to have original biological material and not contamination, also contained five percent modern carbon! See more at Round Four of our RSR debate with atheist AronRa. We have a young earth!
* Earth's Decaying Magnetic Field Affects 14C Dating: Long-term, authoritative, and worldwide measurements show that the Earth's magnetic field is decaying rapidly (as NASA's data shows is also true of Mercury). Our planet's more powerful magnetic field in the past better shielded Earth from cosmic rays, resulting in less Carbon-14 production. This means that carbon ages of specimens from past millennia and even from only centuries ago need to be adjusted downward. So apart from adjustment for the exponentially decaying magnetic field, specimens are therefore younger than their radiocarbon age indicates. For, living with a stronger field, plants and animals absorbed less radiocarbon. So, in addition to the evidence for rapid radioactive decay at the time of the global flood, the Earth's decaying magnetic field means that specimens are generally younger than claimed by Carbon-14 dating. This effect may be significant going back even only to the time of Christ, and might also be a factor in why 14c dating of the artifacts of the Pharaohs were not as helpful as had been hoped for in resolving debates in chronology among Egyptologists.
* Amber 14C Dating Prediction: RSR predicts that even allegedly 300-million-year-old amber will show significant quantities of modern carbon. See more at RealScienceRadio.com/predictions.
* AMS' Improved Accuracy Reduces Dates: Old-earth scientists are not eager to report that when AMS dates the same materials that were already dated by older, less-precise radiometric dating techniques, the new AMS dates are usually 10–1000 times younger. So it is left to creationists to make sure this footnote in science history is not forgotten, including Walt Brown, Paul Giem, and John Baumgardner.
* Origin of Earth's Radioactivity: The scientific community has now faslified its own supernova theory for the origin of elements heavier than iron. And any suggestion, including the neutron star merger hypothesis, that proposes production of such elements in deep space is a non-starter and falsified by the solar system's non-uniform distribution of isotopes. Therefore, the last theory standing for the origin of radioactivity, and the one that explains the physical evidence and has extensive experimental support from laboratory science, is Walt Brown's creation-based Hydroplate Theory as described at rsr.org/radioactivity. As a former on-air guest (rsr.org/david-willis) has pointed out in private correspondence, Dr. Brown's theory for the origin of Earth's crustal radioactivity intrinsically explains the unreliability of heavy element radiometric dating of Earth rocks while recognizing Carbon 14 dating as relatively accurate (adjusting for the old earthers' erroneous assumption, see above, that 14c levels have been constant over the past 100,000 years). In the HPT, what appears to be accelerated radioactive decay applies to elements in the crust and inside the subterranean water chamber (beneath the firmament). This effect would not have applied to an element in the atmosphere, such as 14c. So as a result of widespread misunderstanding of Earth's history, old-earth geologists contend with infamously contradictory million- and billion-year radiometric dates, whereas carbon dating is not subject to the same extreme physical forces that play havoc with other radiometric dating methods.
* Three Related Lines of Evidence Shoring Up the Young Earth Interpretation: The interaction between dinosaur soft tissue, unracemized left-handed amino acids, and Carbon 14 must all be explained to understand the true age of the geologic column. (1) Significant amounts of radiocarbon in diamonds, a mosasaur bone, dinosaur fossils, marble, fossilized wood, natural gas, coal, oil and other petroleum products, deep groundwater, geological graphite, and Mesozoic-layer limestone. (2) There's mostly unracemized left-handed amino acids in chert and dinosaur eggshells. They they would have rapidly racemized (decayed, sort of) in only thousands of years even under Earth's most favorable conditions, to a randomized 50/50 right-to-left ratio. (3) There's flexible and even transparent blood vessels with more than 100 journal reports of original biomaterial fossils including entire cells and even T. rex and hadrosaur DNA (with a half-life of ~521 years) in dinosaur soft tissue fossils. Many such lines of evidence (see some fun examples at rsr.org/not-so-old-things) undermine the claim by old-earth geologists that the plentiful 14c in "ancient" specimens must come from contamination or neutron capture (see above), and this evidence helps to confirm the young earth interpretation of the data.
* Left-handed Amino Acids: Scientists have been surprised to find primarily left-handed (i.e., unracemized) amino acids in specimens allegedly hundreds of millions of years old. Once an organism dies, its amino acids begin to return to their inanimate, 50/50 ratio of right- and left-handedness. Duane Gish, highly qualified to address the topic, points out that factors affecting a specimen, including variation in temperature and especially pH (acidity vs. alkalinity), can dramatically speed up the rate of racemization. Merely giving the old-age assumption the benefit of the doubt, however, by assuming the lowest reasonable alkalinity and temperatures for the life of the specimen, retains amino acid racemization as a powerful tool for falsifying million and billion year dates, especially when combined with extant soft tissue and plentiful radiocarbon. See more at RSR's List of Dinosaur Soft Tissue Papers.
Today’s Resources: At rsr.org/store you can get the Spike Psarris DVD What You Aren't Being Told About Astronomy! Have you browsed through our Science Department in the KGOV Store? You just might love it!
* Debate Announcement: Bob has just posted the final round conclusion to our RSR debate with anti-creationist AronRa on the British atheist website, League of Reason. For links to the on-air and written debates, round by round, see kgov.com/AronRa. And click here for Bob's final round post!
* YouTube Video of the RSR Offer to Jack Horner: Steven Spielberg had famed paleontologist Jack Horner on the set as a technical advisor during the filming of all three Jurassic Park blockbuster movies. This YouTube video presents the Real Science Radio phone call by which we offered Jack Horner a grant of $23,000 to carbon date either their Wankel T. rex or their pregnant (at the time of its death) B. rex.

Student Assignment: Find out if carbon date estimates for ancient trees consistently date older than estimates from counting the tree rings (dendrochronology).